Research Interests

P. Schienbein. J. Chem. Theory Comput 2023, 19, 705
Predictive Vibrational Spectroscopy
Vibrational Spectroscopy is an invaluable and all-abundant tool providing critical insights in the structural dynamics of atoms and molecules in condensed phases, particularly on the delicate interplay of solute and solvent molecules. It also builds a bridge between theory and experiment. Simulations can be validated against experimental measurements and contribute detailed atomistic understanding which is often missing from experiments alone. Calculating predictive vibrational spectra is, however, computationally demanding and limited to small system sizes. We are therefore interested in accelerating their calculation by developing new or leveraging existing machine learning models. Recently, we have introduced the atomic polar tensor as a target property for a machine learning model, offering a rigorous path towards accurate IR spectroscopy [J. Chem. Theory Comput., 2023]. The corresponding atomic polar tensor neural network is available on [Github].
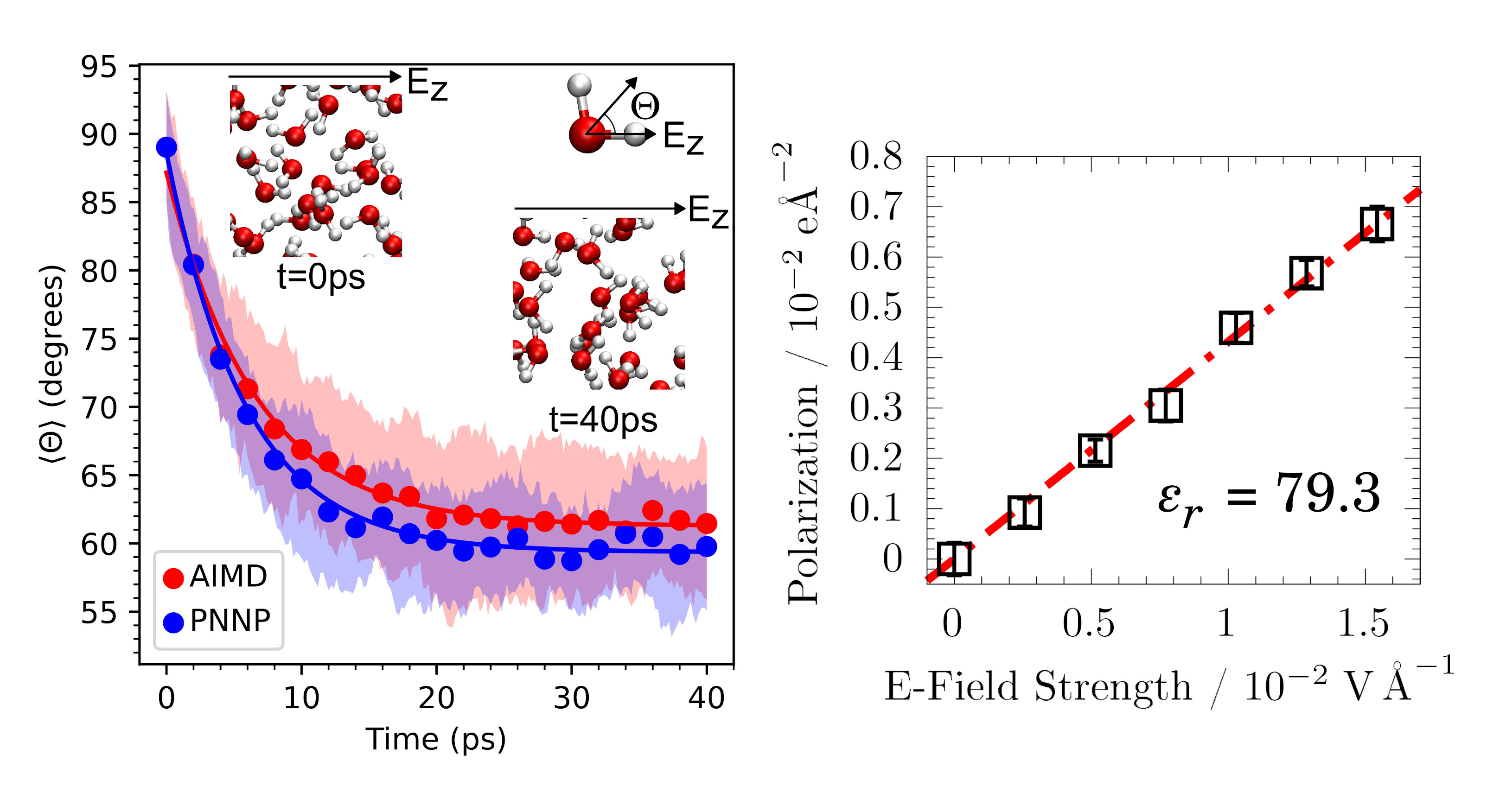
Electric Field Simulations
Electric fields are omnipresent in nature and technology. They play a central role in a myriad of electronic and energy conversion devices, including field effect transistors, (super-)capacitors, batteries and solar cells. In chemistry, electric fields can be used to steer selectivities in catalysis and to control reactivities. Finally, they are present at electrified interfaces, which are currently a major research topic in theoretical chemistry. We have recently introduced a perturbative approach to accurately treat external electric fields in machine learning molecular simulations based on the atomic polar tensor [Nat. Commun., 2024]. The code to conduct such simulations is available on [Github]. Being able to conduct those simulations is a major ingredient of our current research, for instance to calculate conductivities in electrolytes which are of crucial importance in battery development.
P. Schienbein and J. Blumberger. Phys. Chem. Chem. Phys. 2022, 24, 15365
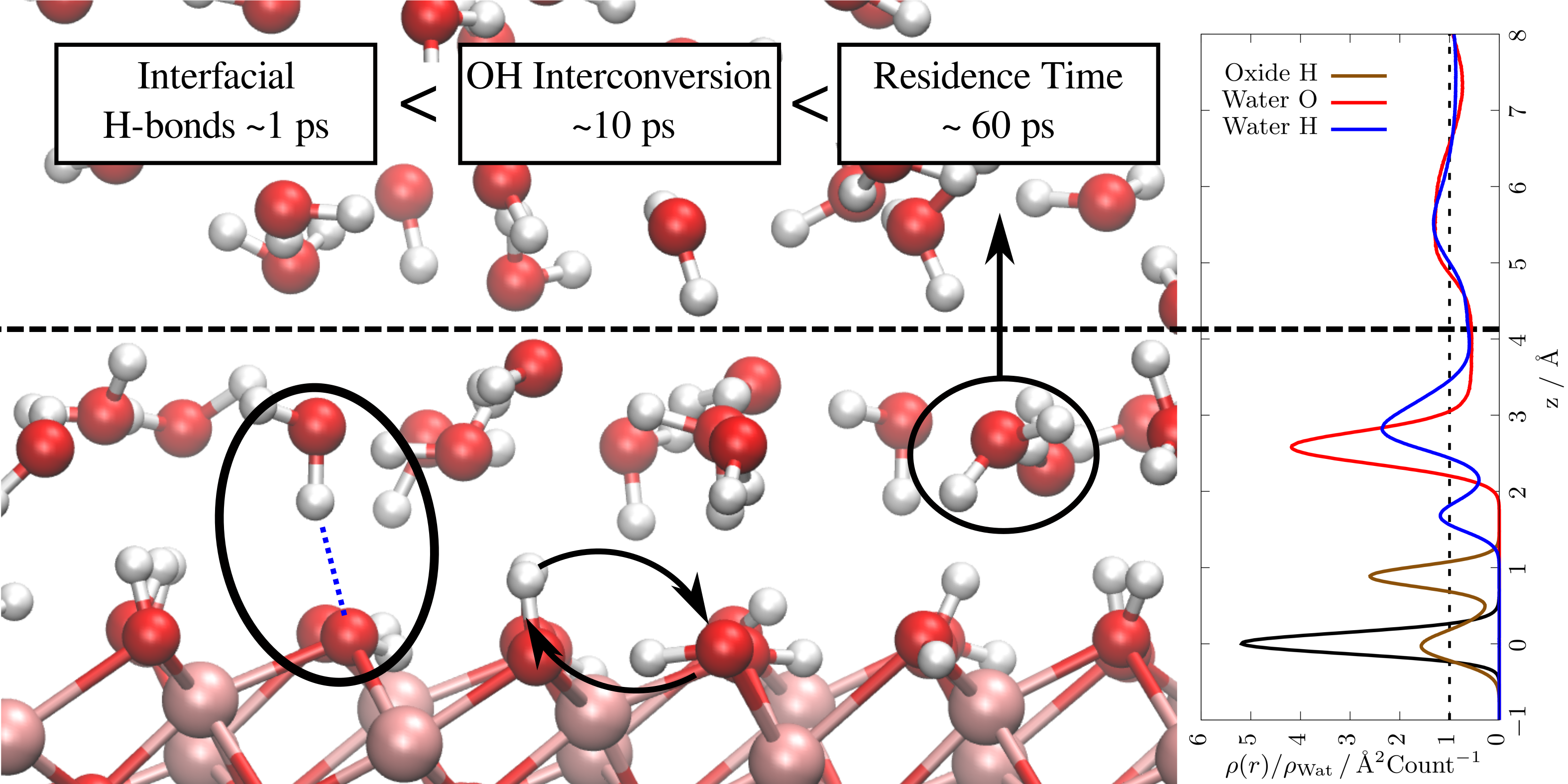
Metal Oxide / Liquid Interfaces
Metal oxide/water interfaces play an important role in biology, catalysis, energy storage, photocatalytic water splitting, or fuels from CO2 (artificial photosynthesis). Our research focuses on using molecular simulations to uncover the solvation dynamics at such interfaces, for example of the hematite/water interface [Phys. Chem. Chem. Phys., 2022]. Machine learning potentials have been an indispensable tool to accelerate these simulations, thereby allowing us to converge dynamical properties which is not possible using brute-force ab initio molecular dynamics. Another complementary aspect are thermodynamic properties, particularly free energy differences which determine the microscopic structure. One example are protonation states which are governed by their corresponding deprotonation free energies. Recently, we have devised a systematic workflow to calculate such free energies using thermodynamic integration from machine learning potentials and active learning [ChemPhysChem, 2024].
Liquids and Solutions
Liquids and solutions are essential to daily life, particularly liquid water (“the matrix of life”1) and aqueous solutions. Understanding solvent-solute interactions is crucial, as solvents actively influence chemical reactions, thermodynamic properties (e.g., melting and boiling points), and conductivities. These interactions also affect protein folding in biological systems and determine the bioavailability of pharmaceuticals. Previously, we studied water along its liquid-vapor phase diagram up to the supercritical phase, demonstrating that the characteristic hydrogen bond network of liquid water largely dissipates under these conditions [Angew. Chem. Int. Ed., 2020] [Science Advances, 2025]. Now, we are expanding our research towards electrolyte solutions and other industrially relevant solvents.